| Highlights | Electrically driven ion adsorption and recovery was demonstrated for butyrate, a key input for bio-based hydrocarbon fuels and chemicals. |
| Motivation | Short-chain carboxylic acids and their derivatives are a platform for the production of bio-based fuels and chemicals. Renewable hydrocarbon biofuels are critically important for sectors that are difficult to electrify, including transportation, which accounts for 26% of total U.S. energy consumption (EIA 2022) and 27% of 2020 greenhouse gas emissions (EPA 2022). To date, however, biofuels have not reached cost parity with conventional petroleum-based fuels due to separation challenges that drive energy consumption, economics, and environmental footprint. The overall objective of this work was to develop electrochemical separations to selectively separate and recover an aqueous carboxylate (i.e., butyrate) because production of this intermediate and subsequent chemocatalytic upgrading has been identified as a potential strategy to achieve biofuel cost targets (Davis et al. 2018). |
| Approach | Capacitive deionization (CDI) is an electrochemical method that does not require a phase transition or chemical reagents. It is intrinsically reversible through modulation of the electric potential and provides a pathway for electrification, which is key to decarbonizing the economy. Furthermore, tunable electrode materials and flexible operation strategies can be used to achieve selective separation. To this end, the Consortium explored redox-based electrochemical separations (RECS) to achieve increased selectivity of target compounds (low molecular weight organic acids) and lower operating potentials. RECS builds on CDI through redox-functionalized electrodes. As such, the technical approach included parallel development of redox-functionalized electrode materials and CDI process development at the bench scale to evaluate separation performance. |
| CDI Process Design | Reversible electrosorption of sodium butyrate was demonstrated using CDI based on scalable electrochemical cell designs. As shown in Figure 1, feed solution is pumped into an electrochemical cell consisting of a pair of porous, conductive electrodes. A potential bias is applied to the electrodes, resulting in salt adsorption and a reduction in effluent conductivity. This is followed by the removal of potential, resulting in the release of the captured ions into a concentrated stream. Adsorption capacity, kinetics, and energy consumption were dependent on operating conditions such as operation time, applied potential, solution concentration, and cell configuration. In addition to demonstrating long-term operation (>1,000 charge/discharge cycles), a supervisory control and data acquisition system was custom-built to enable automated, continuous CDI experimentation, operation, and data collection with synchronous control of process variables. |
| Redox-Active Materials | Redox-functionalized electrodes were fabricated through the coating of carbon-based substrates, resulting in characteristic oxidation-reduction peaks in cyclic voltammetry. |
| Economics and Sustainability | Cost-benefit analysis indicated that the electrode cost and capacity most influence the separation cost. Biorefinery-level techno-economic analysis indicated that the butyrate concentration, its recovery, and the associated energy consumption are critical to meeting the fuel selling price target. Life cycle assessment was conducted using the GREET® model. Integrating CDI for post-fermentation for product recovery could reduce life-cycle greenhouse gas (GHG) emissions of biofuel by 50% or more in comparison to fossil diesel. |
| Figures |  Figure 1. Schematic of CDI operation (left) and experimental data for multiple charge/discharge cycles demonstrating reversible extraction and recovery of sodium butyrate (right). 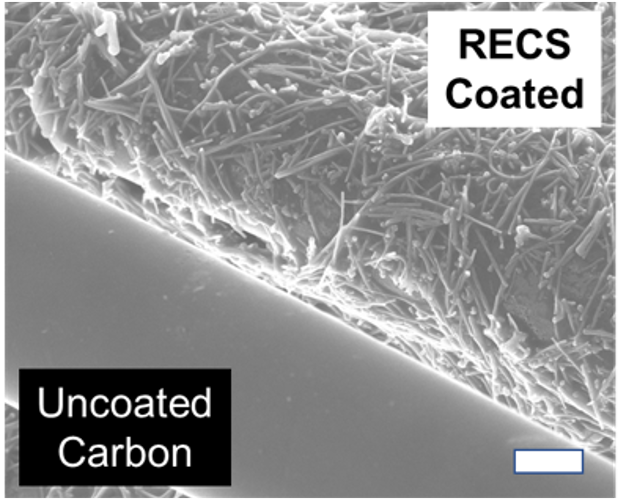 Figure 2. Scanning electron micrograph (scale bar: 2.5 μm) of uncoated and coated carbon fibers. |
